Last Updated on July 24, 2024 by admin
Introduction of Myhep Dvir Tablets:
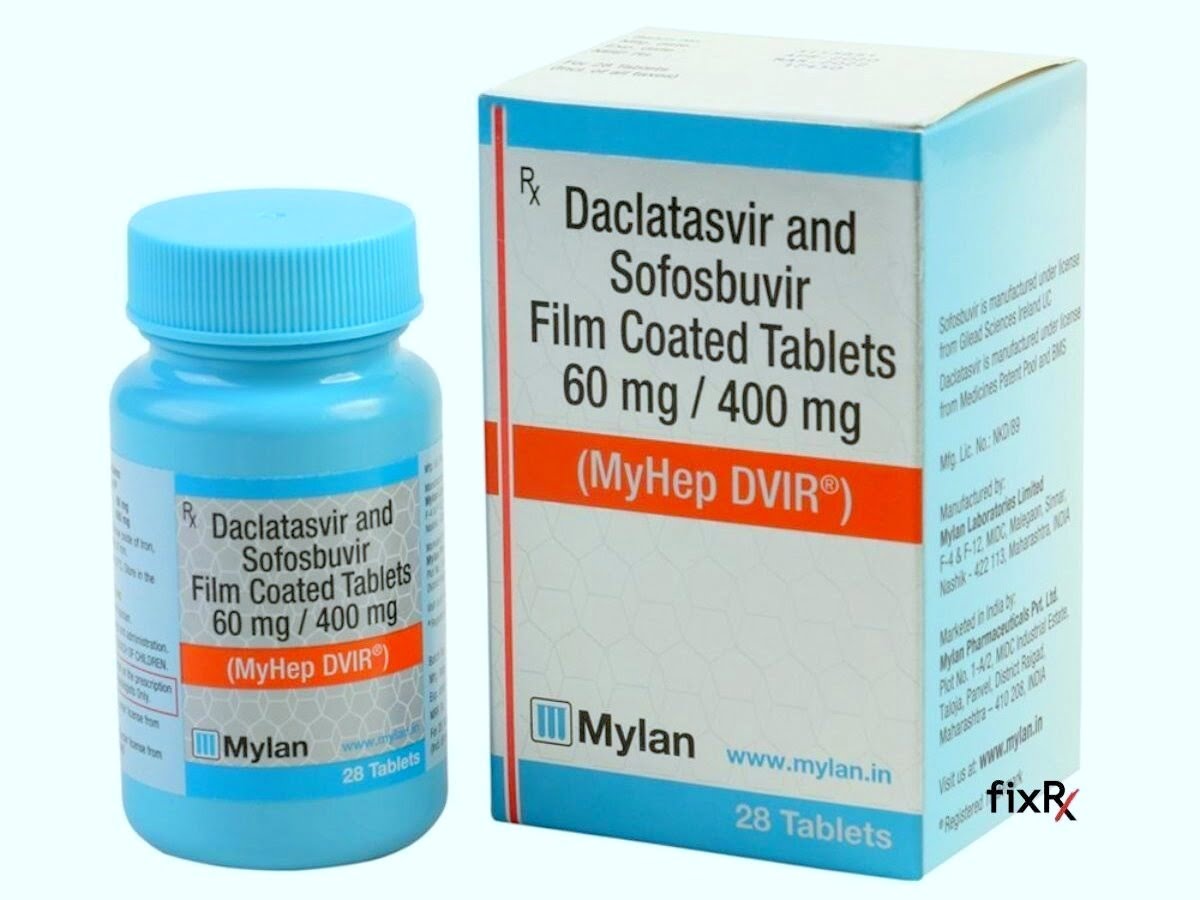
Myhep Dvir Tablets are an essential pharmaceutical product used primarily for the treatment of specific medical conditions. These tablets are a combination medication, consisting of the active ingredients Sofosbuvir and Daclatasvir, which are antiviral drugs. Their primary use is in the management and treatment of chronic Hepatitis C virus (HCV) infection. Hepatitis C is a significant global health concern, affecting millions of individuals worldwide, and the introduction of Myhep Dvir Tablets has marked a pivotal advancement in its treatment.
The unique synergy of Sofosbuvir and Daclatasvir in Myhep Dvir Tablets works by inhibiting the replication of the Hepatitis C virus within the body. Sofosbuvir is a nucleotide polymerase inhibitor, while Daclatasvir acts as an NS5A inhibitor. Together, they disrupt the virus’s lifecycle, effectively reducing the viral load in patients and, in many cases, leading to a sustained virologic response (SVR), which is indicative of a successful treatment outcome. This combination has proven to be highly effective across various genotypes of the Hepatitis C virus, making it a versatile and valuable option in antiviral therapy.
The development and approval of Myhep Dvir Tablets have had a substantial impact on the medical field, providing a more efficient and less invasive treatment option compared to previous therapies. Prior to the advent of such combination medications, Hepatitis C treatment often involved lengthy and arduous regimens with lower success rates and more severe side effects. Myhep Dvir Tablets, with their simplified dosing schedule and improved tolerability, have significantly enhanced patient compliance and quality of life.
In summary, Myhep Dvir Tablets represent a critical advancement in the treatment of chronic Hepatitis C. By effectively combining two potent antiviral agents, they offer a promising solution for patients, contributing to better health outcomes and advancing the fight against this pervasive disease.
USES OF MYHEP DVIR TABLET
BENEFITS OF MYHEP DVIR TABLET
Uses of Myhep Dvir Tablets
Myhep Dvir Tablets are primarily prescribed for the treatment of chronic hepatitis C, a liver infection caused by the hepatitis C virus (HCV). This medication is a combination of two antiviral agents, sofosbuvir and daclatasvir, which work synergistically to inhibit the replication of the virus within the body. By targeting different stages of the viral lifecycle, Myhep Dvir Tablets effectively reduce the viral load, leading to a significant decrease in HCV presence and activity.
The primary benefit of using Myhep Dvir Tablets for hepatitis C patients is the potential for achieving a sustained virologic response (SVR). SVR is considered a cure for hepatitis C, as it indicates that the virus is undetectable in the patient’s blood for a prolonged period post-treatment, typically 12 weeks. Patients achieving SVR are likely to experience marked improvements in liver health and a reduction in liver-related complications.
In addition to its role in treating chronic hepatitis C, Myhep Dvir Tablets may also be prescribed for patients with coexisting conditions, such as HIV. The dual antiviral action of the medication makes it a versatile option for managing complex cases where multiple viral infections are present. This comprehensive treatment approach helps in reducing the overall disease burden and improving the quality of life for affected individuals.
Another important aspect of Myhep Dvir Tablets’ use is in the context of liver transplantation. Patients who have undergone liver transplantation due to hepatitis C-related liver damage can benefit from this medication to prevent reinfection of the new liver. By maintaining an undetectable viral load, Myhep Dvir Tablets play a crucial role in the long-term success of liver transplants and the overall health of transplant recipients.
Overall, Myhep Dvir Tablets offer a promising solution for patients suffering from chronic hepatitis C and related conditions. The effectiveness of the medication in achieving SVR and improving liver function underscores its significance in contemporary antiviral therapy.
Directions for Use
Proper administration of Myhep Dvir Tablets is crucial to ensure the effectiveness of the medication and to minimize potential side effects. It is essential to adhere strictly to the dosage recommendations provided by your healthcare provider. Typically, Myhep Dvir Tablets are taken once daily, with or without food. It is advised to take the tablet at the same time each day to maintain consistent levels of the medication in your bloodstream.
The prescribed dosage for Myhep Dvir Tablets may vary depending on the specific condition being treated and the patient’s overall health. Always follow the exact instructions given by your doctor and do not alter the dosage without consulting them first. Swallow the tablet whole with water; do not crush, chew, or break it, as this can affect the way the medication is absorbed in your body.
Adherence to the prescribed schedule is paramount. Missing doses or taking the medication inconsistently can reduce its efficacy and potentially lead to resistance. If you happen to miss a dose of Myhep Dvir Tablets, take it as soon as you remember. However, if it is almost time for your next scheduled dose, skip the missed dose and resume your regular dosing schedule. Do not double up on doses to make up for a missed one, as this can increase the risk of adverse effects.
Additionally, storing Myhep Dvir Tablets correctly is important to maintain their potency. Keep the medication in its original packaging, away from moisture and direct sunlight, and store it at room temperature. Always keep it out of reach of children and pets.
By following these guidelines, you can help ensure that you are taking Myhep Dvir Tablets effectively and safely. If there are any uncertainties or side effects experienced, contact your healthcare provider immediately for guidance.
SAFETY ADVICE
Alcohol

Pregnancy

Breast feeding

Driving
Kidney
Liver
Benefits of Myhep Dvir Tablets
Myhep Dvir Tablets have garnered significant attention for their remarkable efficacy in treating specific medical conditions. Primarily used in the treatment of Hepatitis C, Myhep Dvir Tablets have demonstrated a high cure rate, substantially improving patient outcomes. Clinical studies have shown that patients using Myhep Dvir Tablets experience a marked reduction in viral load, leading to a sustained virologic response (SVR), which is often equated with a cure. Notably, a pivotal study revealed that over 95% of patients achieved SVR after a 12-week course of Myhep Dvir therapy.
One of the standout benefits of Myhep Dvir Tablets is the rapid alleviation of symptoms associated with Hepatitis C. Patients often report significant improvements in fatigue, nausea, and liver-related symptoms within a few weeks of commencing treatment. This improvement in symptoms not only enhances the quality of life but also facilitates a quicker return to normal daily activities. Moreover, the use of Myhep Dvir Tablets has been associated with a decrease in the risk of developing liver-related complications such as cirrhosis and liver cancer, further underscoring its long-term benefits.
In addition to clinical studies, numerous patient testimonials corroborate the efficacy of Myhep Dvir Tablets. Patients frequently highlight the ease of use and minimal side effects compared to other treatment options. For instance, a patient testimonial featured in a prominent medical journal detailed a positive treatment journey, emphasizing the simplicity of the regimen and the significant health improvements experienced. Such testimonials are invaluable, providing real-world evidence that supports clinical data.
Overall, Myhep Dvir Tablets offer a comprehensive solution for managing Hepatitis C, ensuring both symptom relief and long-term health benefits. With strong clinical backing and positive patient experiences, Myhep Dvir Tablets stand out as a reliable and effective treatment option in the medical community.
Storage Instructions
Proper storage of Myhep Dvir Tablets is essential to maintain their efficacy and ensure patient safety. These tablets should be stored at a controlled room temperature ranging between 20°C to 25°C (68°F to 77°F). It is imperative to keep the tablets in their original packaging until they are ready to be taken, as this helps protect them from exposure to elements that could degrade their potency.
One of the critical factors to consider is protection from light and moisture. Myhep Dvir Tablets should be stored in a dry place and away from direct sunlight. Exposure to excessive moisture and light can lead to a breakdown of the active ingredients, rendering the medication less effective. Therefore, it is advisable to keep the medication in a tightly sealed container, preferably one that is resistant to both light and moisture.
In addition to proper storage, it is crucial to follow guidelines for the disposal of expired or unused Myhep Dvir Tablets. These tablets should not be flushed down the toilet or poured into a drain unless specifically instructed to do so. The best approach to disposing of unused medication is through a drug take-back program. Many communities offer these programs to safely dispose of medications, ensuring they do not pose a risk to others or the environment. If such a program is unavailable, consult a pharmacist for alternative disposal methods.
By adhering to these storage instructions, you can help ensure that Myhep Dvir Tablets remain effective and safe for use throughout their intended shelf life. Proper storage and disposal not only safeguard the medication’s integrity but also contribute to a safer home environment by preventing accidental ingestion or misuse.
Medicinal Benefits and Mechanism of Action
Myhep Dvir Tablets offer a range of medicinal benefits primarily through their active ingredients, Sofosbuvir and Daclatasvir. These compounds work synergistically to treat chronic hepatitis C virus (HCV) infections. The therapeutic efficacy of Myhep Dvir is primarily attributed to the specific mechanisms of action of these active ingredients.
Sofosbuvir, a nucleotide analog polymerase inhibitor, targets the RNA-dependent RNA polymerase, which is crucial for the viral replication process. By inhibiting this enzyme, Sofosbuvir effectively halts the replication of the HCV RNA, thereby reducing the viral load in the patient’s body. This interruption in the replication cycle is a critical step in controlling and eventually eradicating the infection.
Daclatasvir, on the other hand, acts as an NS5A inhibitor. NS5A is a non-structural protein essential for the replication and assembly of HCV. Daclatasvir binds to this protein, disrupting the formation of the replication complex. This disruption further inhibits the replication of the virus, enhancing the antiviral activity initiated by Sofosbuvir. The combination of Sofosbuvir and Daclatasvir in Myhep Dvir Tablets ensures a comprehensive blockade of viral replication at multiple stages, thereby maximizing the therapeutic outcomes.
The medicinal benefits of Myhep Dvir Tablets extend beyond viral suppression. By significantly lowering the HCV RNA levels, these tablets contribute to the improvement of liver function and a reduction in liver inflammation and fibrosis. This not only alleviates the symptoms associated with chronic HCV infection but also diminishes the risk of long-term complications such as liver cirrhosis and hepatocellular carcinoma.
Moreover, the dual-action mechanism of Myhep Dvir enhances the likelihood of achieving sustained virologic response (SVR), which is indicative of a successful treatment outcome. Patients reaching SVR are considered cured, as the virus remains undetectable in the blood for extended periods, typically 12 weeks post-treatment. This underscores the importance of Myhep Dvir Tablets in the management and eventual eradication of hepatitis C.
Drug Warnings and Interactions
Myhep Dvir Tablets are a combination antiviral medication used to treat chronic Hepatitis C infection. However, understanding potential warnings and interactions is crucial for patient safety. It is essential to consult healthcare providers before starting Myhep Dvir Tablets to ensure that the treatment is appropriate for the individual’s medical condition and history.
Contraindications: Myhep Dvir Tablets are contraindicated in patients with severe renal impairment or end-stage renal disease. Additionally, individuals with known hypersensitivity to any component of the medication should avoid its use. Patients with a history of significant liver issues, other than Hepatitis C, should exercise caution and consult a healthcare provider.
Precautions for Specific Populations: Pregnant women should not use Myhep Dvir Tablets unless absolutely necessary, as the effects on fetal development are not entirely known. Women of childbearing potential should use effective contraception during treatment and for a period after completing the therapy. Elderly patients may experience increased sensitivity to the drug, and thus, require closer monitoring during treatment.
Drug Interactions: Myhep Dvir Tablets can interact with various medications, potentially altering their effectiveness or increasing the risk of adverse effects. Notably, concurrent use of Myhep Dvir Tablets with drugs such as amiodarone, rifampin, and certain anticonvulsants can lead to significant interactions. This medication can also interact with herbal supplements like St. John’s Wort, which may reduce its efficacy. Therefore, it is imperative to provide healthcare providers with a comprehensive list of all medications and supplements being taken.
Patients are advised to follow healthcare providers’ recommendations and attend regular follow-up appointments to monitor the effectiveness and safety of Myhep Dvir Tablets. Reporting any unusual symptoms or side effects promptly can help manage potential risks and ensure the best possible outcomes from the treatment.
SIDE EFFECTS OF MYHEP DVIR TABLET
Common side effects of Myhep Dvir
- Fatigue
- Nausea
- Headache
Side Effects of Myhep Dvir Tablets
Myhep Dvir Tablets, like any medication, may cause side effects in certain individuals. Understanding these potential reactions is crucial for users to manage their health effectively. Side effects can be categorized into common, less common, and severe. Each category should be monitored with varying degrees of attention.
Common side effects of Myhep Dvir Tablets include headaches, nausea, fatigue, and dizziness. These reactions are typically mild and may diminish as the body adjusts to the medication. If these symptoms persist or become bothersome, consulting a healthcare professional is advisable.
Less common side effects might involve skin rashes, itching, or changes in taste. These reactions, though not frequent, still warrant attention. It is recommended to inform your doctor if you experience any of these symptoms, as they may require adjustments to your treatment plan or additional interventions.
Severe side effects are rare but can occur. These include severe allergic reactions, such as swelling of the face, lips, or throat, difficulty breathing, and chest pain. Additionally, unusual bleeding or bruising, dark urine, and yellowing of the skin or eyes may indicate serious liver problems. Immediate medical attention is crucial in these cases to prevent further complications.
If you encounter any side effects while taking Myhep Dvir Tablets, it is important to document them and communicate with your healthcare provider promptly. They can provide guidance on managing these reactions and determine whether the medication should be continued or adjusted. Always follow your doctor’s instructions and never alter your dosage without professional advice.
frequently asked questions (FAQs) about Myhep Dvir tablets:
General Information
Q: What is Myhep Dvir Tablets?
A: Daclatasvir and Sofosbuvir are antiviral medications used together to treat chronic hepatitis C virus (HCV) infection. This combination is effective in eliminating the virus from the body.
Q: What are the brand names for Daclatasvir/Sofosbuvir Tablets?
A: Daclatasvir is marketed under the brand name Daklinza, and Sofosbuvir is marketed under the brand name Sovaldi. When used in combination, they may be known by different brand names depending on the region, like india Myhep Dvir Tablets
Dosage and Administration
Q: What is the typical dosage for Myhep Dvir Tablets?
A: The typical dosage is Daclatasvir 60 mg once daily and Sofosbuvir 400 mg once daily. The exact dosage and duration depend on the specific genotype of the HCV, the presence of cirrhosis, and prior treatment history.
Q: How should I take Myhep Dvir Tablets?
A: Take the medication exactly as prescribed by your doctor. Swallow the tablets whole with or without food. It is important to take them at the same time every day to maintain consistent levels in your bloodstream.
Side Effects
Q: What are the common side effects of Myhep Dvir Tablets?
A: Common side effects may include headache, fatigue, nausea, and insomnia. Most side effects are mild and temporary.
Q: Are there any serious side effects I should be aware of?
A: Serious side effects are rare but can occur. They may include allergic reactions, severe liver problems, or heart issues. Contact your healthcare provider immediately if you experience symptoms like severe dizziness, fainting, or jaundice.
Drug Interactions
Q: Can Myhep Dvir Tablets interact with other medications?
A: Yes, Daclatasvir and Sofosbuvir can interact with other medications, which may affect how they work or increase the risk of side effects. Inform your doctor about all other medications, supplements, and herbal products you are taking.
Q: Are there any medications that should be avoided while taking Myhep Dvir Tablets?
A: Avoid taking medications like rifampin, carbamazepine, and St. John’s wort, as they can reduce the effectiveness of Myhep Dvir Tablets. Your doctor will provide a complete list of medications to avoid.
Efficacy
Q: How effective is Myhep Dvir Tablets in treating hepatitis C?
A: Clinical trials have shown that the combination of Daclatasvir and Sofosbuvir is highly effective, with cure rates of over 90% for many HCV genotypes. The success rate depends on various factors, including the HCV genotype and the presence of cirrhosis.
Q: How long will I need to take Myhep Dvir Tablets?
A: The typical treatment duration is 12 to 24 weeks, depending on the HCV genotype, treatment history, and presence of cirrhosis.
Storage and Handling
Q: How should I store Myhep Dvir Tablets tablets?
A: Store the tablets at room temperature, away from moisture, heat, and light. Keep them in their original container and out of reach of children.
Additional Information
Q: Can pregnant or breastfeeding women take Myhep Dvir Tablets?
A: The safety of Myhep Dvir Tablets during pregnancy and breastfeeding is not well established. If you are pregnant, planning to become pregnant, or breastfeeding, discuss the potential risks and benefits with your doctor.
Q: What should I do if I miss a dose?
A: If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose and take your next dose at the regular time. Do not take two doses at the same time.
Q: What should I do in case of an overdose?
A: In case of an overdose, seek emergency medical attention or contact a Poison Control Center immediately. Symptoms of overdose may include severe dizziness or fainting.
Always consult your healthcare provider for personalized medical advice and before starting or stopping any medication.
All substitutes for Myhep Dvir Tablet
References:
- Bristol-Myers Squibb Company, US Food and Drug Administration, [Revised on Feb 2017] [Accessed on 12th May 2023], https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/206843s006lbl.pdf
- Gilead Sciences Ireland UC, European Medical Agency (ema), [Revised on Sept 2018] [ Accessed on 12th May 2023], https://www.ema.europa.eu/en/documents/product-information/sovaldi-epar-product-information_en.pdf
- Gilead Sciences, US Food, and Drug Administration, [ Revised on August 2015] [ Accessed on 12th May 2023], https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204671s002lbl.pdf