Last Updated on March 22, 2023 by admin
Buy Qvir Kit Tablet online
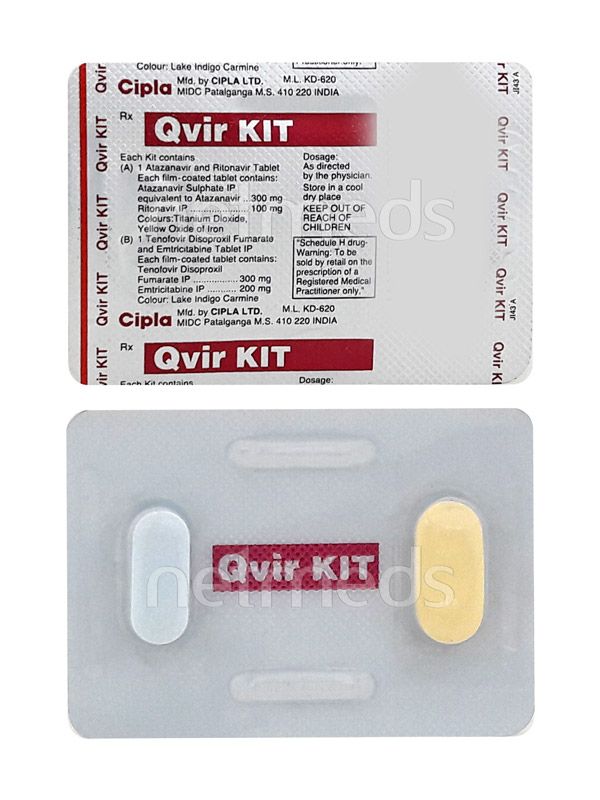
About Qvir Kit Tablet
SALT COMPOSITION
Tenofovir disoproxil fumarate (300mg) + Emtricitabine (200mg) + Atazanavir (300mg) + Ritonavir (100mg)
Qvir Kit is a combination of four antiretrovirals. It is prescribed to treat HIV (human immunodeficiency virus) infection. It boosts up the immunity to fight against HIV to manage or treat AIDS (acquired immunodeficiency syndrome).
Qvir Kit restricts the growth of HIV in the body and reduces the risk of getting HIV-related complications to improve the lifespan of an individual. The medicine should be taken with food. Taking these medicines regularly at the same time increases their effectiveness. A dose of this medicine should not be missed as it can affect your recovery. It is important to complete the full course of the treatment until your doctor advises you to stop it.
USES OF QVIR KIT
- Treatment of HIV infection
BENEFITS OF QVIR KIT
- In Treatment of HIV infection
Qvir Kit controls HIV infection by preventing the virus from multiplying in the body. It also helps your immnune system to work better and may decrease your chance of developing HIV-related illnesses such as AIDS. This ensures that you live a long and healthy life. Avoid skipping doses and follow your doctor’s instructions carefully to get maximum benefit. Taking Qvir Kit along with practicing safer sex and making other lifestyle changes may decrease the risk of transmitting the HIV virus to other people.
SIDE EFFECTS OF QVIR KIT
Most side effects do not require any medical attention and disappear as your body adjusts to the medicine. Consult your doctor if they persist or if you’re worried about them
Common side effects of Qvir
- Nausea
- Vomiting
- Diarrhea
- Stomach pain
- Headache
- Depression
- Insomnia (difficulty in sleeping)
- Taste change
- Skin rash
- Peripheral neuropathy (tingling and numbness of feet and hand)
- Increased blood lipid level
- Increased liver enzymes
HOW TO USE QVIR KIT
Take this medicine in the dose and duration as advised by your doctor. Check the label before use. Qvir Kit is to be taken with food.
HOW QVIR KIT WORKS
Qvir Kit is a combination of four antiviral medicines: Tenofovir disoproxil fumarate, Emtricitabine, Atazanavir and Ritonavir. Tenofovir disoproxil fumarate and Emtricitabine work by preventing HIV (virus) from multiplying, thereby reducing the amount of virus in your body. They also increase the CD4 cell (white blood cells that protect against infection) count in your blood. Atazanavir and Ritonavir work by interfering with an enzyme (protease), which is required by HIV-infected cells to make new viruses.
SAFETY ADVICE
warnings
![]() Alcohol
Alcohol
CAUTION
Caution is advised when consuming alcohol with Qvir Kit. Please consult your doctor.
warnings
![]() Pregnancy
Pregnancy
SAFE IF PRESCRIBED
Qvir Kit is generally considered safe to use during pregnancy. Animal studies have shown low or no adverse effects to the developing baby; however, there are limited human studies.
warnings
![]() Breast feeding
Breast feeding
SAFE IF PRESCRIBED
Qvir Kit is probably safe to use during breastfeeding. Limited human data suggests that the drug does not represent any significant risk to the baby.
warnings
![]() Driving
Driving
UNSAFE
Qvir Kit may decrease alertness, affect your vision or make you feel sleepy and dizzy. Do not drive if these symptoms occur.
warnings
![]() Kidney
Kidney
SAFE IF PRESCRIBED
Qvir Kit is probably safe to use in patients with kidney disease. Limited data available suggests that dose adjustment of Qvir Kit may not be needed in these patients. Please consult your doctor.
warnings
![]() Liver
Liver
SAFE IF PRESCRIBED
Qvir Kit is probably safe to use in patients with liver disease. Limited data available suggests that dose adjustment of Qvir Kit may not be needed in these patients. Please consult your doctor.
warnings
![]() CHILDREN CAUTION
CHILDREN CAUTION
UNSAFE
Qvir Kit Tablet is not recommended for use in children and adolescents below 18 years.
![]() Qvir Kit Tablet Habit Forming : NO
Qvir Kit Tablet Habit Forming : NO
![]() expert advice
expert advice
WHAT IF YOU FORGET TO TAKE QVIR KIT?
If you miss a dose of Qvir Kit, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular schedule. Do not double the dose.
- Qvir Kit must be taken with food at the same time every day.
- Do not skip any doses and finish the full course of treatment even if you feel better. Stopping it early may make the infection come back and harder to treat.
- It is advisable to drink plenty of fluids and a healthy diet while taking the medicine.
- It may cause dizziness. Do not drive or do anything that requires mental focus until you know how medicine affects you.
- Avoid consuming alcohol while taking medication as it may cause increased side effects.
![]() FOOD AND DRINK
FOOD AND DRINK
- Eat a healthy and balanced diet. Eat vitamin and nutrient-rich food such as dark-green, yellow, orange, and red vegetables and fruit as it helps to boost your immune system. Option for lean protein and whole grains.
- Avoid eating raw meat and eggs. Consume properly boiled and cooked meat, poultry, or seafood.
- Eat bland and low-fat foods and avoid spicy or oily foods if you experience nausea or vomiting.
- Try to reduce emotional and physical stress by spending time with family or whatever makes you happy.
- Drink more fluids and avoid alcoholic beverages to prevent dehydration.
- Wash your hands properly before and after eating.
- Make sure you sleep at least 8 hours a day.
substitutes







Shreekant –
Thanks sir direct net rate availability
Prakash zaa –
best price
admin –
thank you, sir